lewis structure for so2|SO2 Lewis Structure : Baguio Learn how to draw the Lewis structure for SO2, a molecule with a central sulfur atom and two oxygen atoms. Follow the steps to count valence electrons, identify the central atom, connect the atoms, distribute the . The Nintendo DS has arguably one of the best lineups of Pokemon games ever, offering quantity, quality, and even variety. Admittedly, not every release was a success story, particularly concerning .
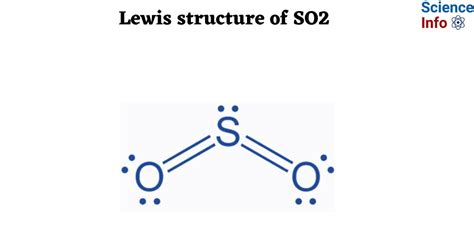
lewis structure for so2,This chemistry video tutorial explains how to draw the lewis structure of SO2 also known as Sulfur Dioxide. It discusses the molecular geometry, bond angle, .

Learn how to draw the Lewis structure for SO2 (sulfur dioxide) with a step-by-step explanation and examples. Find out how to use formal charges, octets, and .Learn how to draw the Lewis structure for SO2, a molecule with a central sulfur atom and two oxygen atoms. Follow the steps to count valence electrons, identify the central atom, connect the atoms, distribute the .
lewis structure for so2Learn how to draw the lewis structure of SO2, a pungent-smelling gas with various industrial uses. Find out the hybridization, molecular geometry, and MO diagram o. Learn how to draw the Lewis structure of SO2 with formal charges, resonance, and expanded octet. Find out the hybridization, bond angles, and molecular geometry of SO2 with examples and diagrams.Learn how to draw the lewis structure of SO2 molecule using VSEPR theory rules and stability criteria. Find out the hybridization, lone pairs, charges and similarities of SO2 with other molecules.Certain cookies and other technologies are essential in order to enable our Service to provide the features you have requested, such as making it possible for you to access . Learn how to draw the lewis dot structure of SO2 (sulfur dioxide) with 6 simple steps and images. Find out the valence electrons, octet rule, formal charge and . Lewis Structure of SO2. A sulfur atom (S) and two oxygen atoms (O) make up the SO2 Lewis structure. The sulfur atom (S) is the center atom, and the two oxygen .
In this video, we'll learn about the Lewis Structure of Sulphur Dioxide - SO2. This structure is key to understanding the chemistry of Sulphur Dioxide. We'll.SO2 Lewis Structure Today in this video, we will determine the Lewis dot structure for sulphur dioxide, having a chemical formula of SO2. It comprises one sulphur atom and two O.
Steps of drawing SO2 lewis structure Step 1: Find the total valence electrons in SO2 molecule. In order to find the total valence electrons in SO2 (sulfur dioxide) molecule, first of all you should know .
For the arrangement HCN, the Lewis structure: H–C\(\equiv\)N: The formal charges work out as follows: For the arrangement HNC, the Lewis structure: H–N\(\equiv\)C: The formal charges work out as follows: Both Lewis structures have a net formal charge of zero, but note that the formal charges on the first structure are all zero! . Here are the steps I follow when drawing a Lewis structure. > 1. Decide which is the central atom in the structure. That will normally be the least electronegative atom ("S"). 2. Draw a skeleton structure in which the other atoms are single-bonded to the central atom: "O-S-O". 3. Draw a trial structure by putting electron pairs around every . Lewis structure of SO2 (or Sulfur dioxide) contains two double bonds between the Sulfur (S) atom and each Oxygen (O) atom. The Sulfur atom (S) is at the center and it is surrounded by 2 Oxygen atoms (O). The Sulfur atom has 1 lone pair and both the Oxygen atoms have 2 lone pairs.What is the structure of SO 2?I have seen two different ways the Lewis Structure is written: The formal charges of the SO 2 with the single bond and a double bond is larger than the SO 2 with two double bonds. So I would assume that the one with two double bonds is the correct structure. There are three resonance structures SO2 (Sulfur dioxide). We start with a valid Lewis structure and then follow these general rules. Note that SO2 is a bit. Steps for drawing the SO2 Lewis structure. Step 1 Calculate the number of valence electrons for S and O. Sulphur and oxygen are both elements of group 16 on the periodic table. Therefore, there are 6 valence electrons in both sulphur and oxygen atoms, so the total valence electrons in the SO2 molecule = valence electrons given by 1 . The formal charge on any atom in a Lewis structure is a number assigned to it according to the number of valence electrons of the atom . {O-SO2}\), and the resonance structures) \(\ce{NO3-}\) (see Example 2 below) \(\ce{CO3^2-}\) (ditto) Notice that some of the resonance structures may not satisfy the octet rule. The \(\ce{NO2}\) .lewis structure for so2 SO2 Lewis Structure The SO2 Lewis Structure also helps in predicting the molecule’s geometry, which is bent due to the presence of two lone pairs on the oxygen atoms. B. Steps in drawing the SO2 Lewis Structure. Drawing the SO2 Lewis .
Question: Draw the Lewis structure for the sulfur dioxide (SO2) molecule. Be sure to include alli resonance structures that satisfy the octet rule Il resonance structures that satisfy the octet rule. Add atom symbol , I .Get the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha. Sulfur dioxide, or SO_2, has two resonance structures which contribute equally to the overall hybrid structure of the molecule. However, a third Lewis structure can be drawn for SO_2 which is more stable in theory, but doesn't quite match experimental data. Let's draw the first two Lewis structures for SO_2. The total number of valence .

A video explanation of how to draw the Lewis Dot Structure for Sulfur Dioxide, along with information about the compound including Formal Charges, Polarity, . In order to calculate the formal charges for SO2 we'll use the equation:Formal charge = [# of valence electrons] - [nonbonding val electrons] - [bonding elec.In conclusion, understanding the SO2 Lewis structure, particularly in the context of mathematics education, is crucial for students to grasp the fundamental principles of chemical bonding. The ability to depict molecular structures using Lewis diagrams enhances students' problem-solving skills and deepens their comprehension of chemical . SO2 lewis structure formal charges:. SO2 lewis structure of total valence electrons 18.Sulfur and oxygen has six electrons. sulfur has six valence electrons, 2 non bonding and 6 bonding electrons. Six bonding electrons divided by 2 , we get 3 electrons. So the Formal charge of sulfur is 6-2-3 =+1. One of the oxygen having formal charge +1.
lewis structure for so2|SO2 Lewis Structure
PH0 · Sulfur dioxide (SO2) Lewis Structure, Hybridization
PH1 · SO2(Sulfur Dioxide) Lewis Structure, Hybridization,
PH2 · SO2 Lewis Structure, Hybridization, Molecular Geometry, and
PH3 · SO2 Lewis Structure
PH4 · SO2 (Sulfur Dioxide) Lewis Structure
PH5 · Lewis Structure of SO2 (sulfur dioxide)
PH6 · Lewis Structure of SO2 (With 6 Simple Steps to Draw!)
PH7 · Lewis Structure of SO2
PH8 · Khan Academy